VBT for Bonding in Coordination Compounds
VBT for Bonding in Coordination Compounds: Overview
This Topic covers sub-topics such as Valence Bond Theory, Overlapping of Atomic Orbitals, Axial Overlapping, Lateral Overlapping, Directional properties of Bonds and, Formation of Sigma and Pi bonds in Ethene
Important Questions on VBT for Bonding in Coordination Compounds
Number of sigma bonds in is :
The number of bonds, bonds and lone pair of electrons in pyridine, respectively.
Which one of the following statement is not correct for sigma-and pi-bonds formed between two carbon atoms?
The sum of number of sigma and pi bonds formed between two carbon atoms in are:
Number and type of bonds between two carbon atoms in CaC2 are
The number of and bonds in allyl isocyanide are
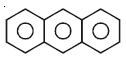
The total number of sigma and pi bonds present in the given anthracene molecule are:
How does oxygen molecule form?
Among the following, which has zero overlapping that leads to non-bonding?
Find out the axis in which nodal plane in the π-bond of is located. (inter-nuclear axis = Z)
In the resonating form of naphthalene molecule, how many sigma and pi bonds are present?
Overlapping leads to the formation of bonding molecular orbital in:
What is the sum of bonds and bonds in a molecule of ?
The number of - and- bonds in hexanedione, respectively are
The combination of orbital that cannot produce non-bonding molecular orbital is (if an inter-nuclear axis is -axis):
If orbital of and orbital of from bond along a particular molecular axis, then which bond will be formed along same molecular axis by combination of orbitals of and atom.
Which of the following gives rise to the formation of bond?
In which of the following back-bonding is possible
When two atoms of hydrogen combine to form a molecule of hydrogen gas, the energy of the molecule is
Which one of the following does not exhibit paramagnetism?
